Does skin cancer drug offer Alzheimer’s hope?
Researchers in the United States have found that an existing cancer drug reverses the symptoms of Alzheimer’s disease in mice, writes Channel 4 News Science Editor Tom Clarke.
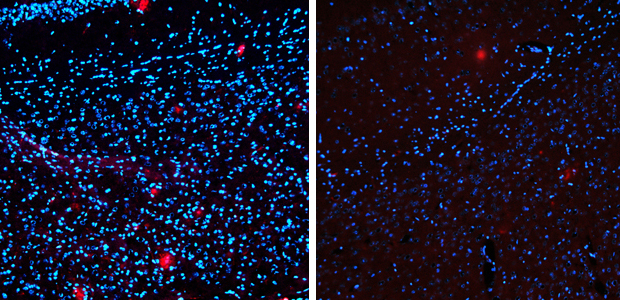
The drug reduced levels of Alzheimer’s-linked amyloid protein (red) by 75 per cent. Left – untreated. Right 14 days after treatment.
The finding, published in the journal Science, opens up a promising new avenue for fighting Alzheimer’s at a time when nearly all drug trials have failed.
The drug, Bexarotene, which is normally used to treat a form of skin cancer, removed the sticky clumps of protein called amyloid in the brains of mice genetically engineered to develop Alzheimer’s symptoms.
“When we saw the effects of the drug on amyloid we were stunned,” Professor Gary Landreth, who led the research at Case Western Reserve University in Ohio, told Channel 4 News.
The drug not only removed up to 75 per cent of the amyloid protein, but also restored some of the behaviours the mice had lost. Because amyloid protein is associated with the progression of Alzheimer’s in people, the finding points at a potentially new target for treating the disease in humans.
What has also excited researchers is the speed at which the drug reduced the amount of protein. Levels of the amyloid protein started falling in treated mice within six hours. In other experiments – like those using vaccines against amyloid protein – it can take months to clear.
Read more: The remote hope for preventing Alzheimer's
Early days for human hope
But for those hoping for a new treatment for Alzheimer’s, the researchers warn the research is at an early stage. “We need to be clear – the drug works well in mouse models of the disease. Our next objective is to ascertain if it acts similarly in humans,” said Landreth.
The researchers told Channel 4 News they are about to begin a trial in healthy human volunteers to see if Bexarotene causes the same biochemical changes in the brain as it does in mice. If so, they can apply for a license to treat Alzheimer’s patients with a form of the drug.
But the drug, in its existing form, may not become a treatment. Though Bexarotene has a “good” safety and side-effect profile according to the US Food and Drug Administration, an Alzheimer’s drug needs to be well tolerated for years, perhaps decades.
The drug development world is littered with drugs that seemed to work on transgenic mice, but didn’t work on people. Prof Derek Hill, Professor of Medical Imaging Science, UCL.
Channel 4 News understands the pharmaceutical industry previously considered the same class of medicines for treating Alzheimer’s but shied away from the idea because they can have harmful side-effects.
The finding comes at an important time for Alzheimer’s research. Many hopeful drug trials have failed, causing many to wonder whether targeting amyloid protein will ever produce results in humans.
Some researchers believe the only way to prevent Alzheimer’s might be to treat it before symptoms emerge, but right now there are no reliable tests that can predict Alzheimer’s.
“The drug development world is littered with drugs that seemed to work on transgenic mice, but didn’t work on people. A programme of clinical trials is now needed to assess whether these potentially promising results translate into an effect on the human disease,” said Professor Derek Hill, Professor of Medical Imaging Science, UCL.
-
Latest news
-
Yungblud launches his own affordable music festival5m

-
Why these Americans want to quit their state9m

-
Company behind infected water outbreak are ‘incompetent’ says local MP5m

-
Israeli forces push deeper into Northern and Southern Gaza4m

-
India’s ‘YouTube election’: Influencers enlisted to mobilise youth vote6m

-




